State of the fertility sector 2020/21
Annual publication on HFEA licensed clinics
Published: November 2021
Download the underlying dataset as .xlsx.
Table of contents
- About this report
- HFEA regulatory response to COVID-19
- Maintaining regulatory oversight
- There were 77 inspections undertaken in 2020/21, taking a hybrid inspection approach
- In 2020/21, 103 clinics were licensed by the HFEA to provide fertility treatment
- The number of incidents in 2020/21 is broadly consistent with previous years
- The number of severe incidents of OHSS reported declined in 2020/21
- A total of 88 patient complaints were received about licensed clinics in 2020/21
About this report
The Human Fertilisation and Embryology Authority (HFEA) is the independent regulator of fertility treatment and human embryo research in the United Kingdom (UK). We aim to ensure that everyone who steps into a fertility clinic, and everyone born as a result of treatment, receives high quality care. We do this by licensing, monitoring and inspecting fertility clinics and providing free, clear and impartial information about fertility treatment, clinics, and egg, sperm, and embryo donation.
State of the Sector is our annual report, which summarises what we have seen through our regulatory work during the year. The report is compiled from information gathered from our inspections throughout the year and from other sources of information including our Register of fertility treatments, incident reports and patient feedback mechanisms. The COVID-19 pandemic had a significant impact on the availability of fertility treatment in 2020/21 and this, combined with the changes we made to our approach to inspection during the year, means that data provided in this report is not directly comparable with previous years and should be interpreted with caution.
In 2019/20, we introduced Clinical Governance Quarterly Updates which provide clinics with detailed insight into non-compliances in a more timely manner to help them improve compliance and prepare for inspections. These updates also contain details of incidents and complaints to ensure clinics can implement any recommendations to help prevent further recurrence. As a result, the State of the Sector report provides a high-level overview of sector-wide compliance, reporting on the absolute number of inspections, non-compliances, incidents, and complaints.
HFEA regulatory response to COVID-19
The NHS was placed under unprecedented strain during the first wave of the pandemic in Spring 2020. In response, the HFEA made the difficult decision on 23 March 2020 to require all clinics to cease fertility treatments by 15 April 2020.i The decision that clinics should close was taken with reference to the following four criteria:
- Guidance regarding the impact of COVID-19 on patient health, including the impact on the health of pregnant women.
- The impact of fertility treatment on the NHS. As fertility treatment is delivered in private clinics as well as NHS facilities, consideration was taken to how private IVF patients could potentially impact the NHS. This included the care of women who become pregnant, and cases requiring emergency referral to the NHS post-fertility treatment.
- The ability of clinics to provide a safe service during the pandemic.
- Government restrictions on social contact and travel.
The date chosen to cease treatments was influenced by guidance on the appropriate provision of fertility services from the relevant UK professional societies, and the decision to cease all non-urgent NHS elective surgery in England by 15 April.
During the time of treatment cessation, only fertility preservation treatments were undertaken (for example for patients about to start chemotherapy). Many clinics during this time had staff redeployed or furloughed and only maintained a skeleton staff to ensure patients were looked after appropriately, and their stored gametes and embryos were kept safe.
As the initial peak of the pandemic subsided, and as new professional guidance was developed to enable clinics to offer safe services during the pandemic, we put in place regulatory arrangements that allowed clinics permission to apply to reopen from 11 May 2020.ii In order to reopen, clinics were required to submit a Treatment Commencement Strategy to the HFEA which demonstrated how their services could be provided in a safe, COVID-19 secure manner. Many clinics were well prepared and were able to recommence treatments quickly, adapting and introducing new ways of delivering their services safely; by 12 June, 94% of private clinics and 76% of NHS clinics had re-opened. Only two clinics did not apply to reopen; one clinic decided to voluntarily revoke their licence and the other clinic had already decided to revoke prior to the pandemic.
Whilst some patients may have been disadvantaged during this short lockdown, treatment numbers quickly increased. The detailed guidance, which was introduced to ensure patients could be treated safely has meant that clinics have been able to continue to offer treatment during all subsequent lockdowns.
Maintaining regulatory oversight
HFEA inspections of clinics were suspended during the national lockdown. However, as the safety of patients and their sperm, eggs and embryos are our priority, we used the lockdown to adapt our regulatory regime and design new ways of working to ensure we could maintain regulatory oversight. Clinics who were due an inspection were assessed using a risk-based approach (RBA), and a desk-based assessment (DBA) process was used to allow continued close oversight of the fertility sector as the pandemic continued. The Authority approved onsite inspections to recommence from November 2020, but as the pandemic continued and tighter Government restrictions were put in place, a risk-based approach continued to be used alongside virtual technologies to minimise the amount of time an inspector physically spent in a clinic. The revised process for the main inspection types is described below.
- Initial Inspections: If appropriate, a DBA of the licence application and virtual inspection were conducted.
- Interim inspections: No interim inspections were conducted at clinics with a four-year licence and where there were no concerns. For clinics where inspectors had concerns, the recommendation was for an inspection to be scheduled at the earliest opportunity when it was deemed safe for inspections to resume.
- Renewal inspections: For all clinics with a four-year licence with no concerns, the recommendation was to extend the licence by one year. For clinics with a four-year licence with significant concerns, or with a licence for less than four years, a DBA was undertaken to review the risks of extending the licence by a further year. If extension was deemed inappropriate, it was noted that an inspection should be scheduled when inspections recommenced.
The inspection resumption strategy consisted of a modified approach of the above, incorporating and adapting pre-existing inspection techniques. Part of the inspection process requires a significant review of clinic documentation, including standard operating procedures, policies, competency assessments and audits; many of these documents can be assessed by a DBA as a first component to inspection. This was incorporated into the DBA/RBA, with further modifications allowing the use of video conferencing and other technologies wherever possible. A modified inspection notebook was sent to clinics 12 weeks prior to their inspection, with all information requested provided electronically to inspectors for review against the requirements. A DBA highlights areas of compliance as well as the risks to compliance. The need to further investigate these risk areas dictated the inspector resources allocated to an onsite inspection. These resources and the approaches used were controlled through each inspection specific risk assessment.
There were 77 inspections undertaken in 2020/21, taking a hybrid inspection approach
Due to the pandemic, 67 inspections were deferred by 12 months. In total in 2020/21, 77 inspections were carried out, of which:
- 17 were completely desk-based
- 32 were a combination of desk-based assessment and onsite visit
- 23 were onsite visits with informal desk-based assessment
- 5 were risk-assessed with no onsite visit
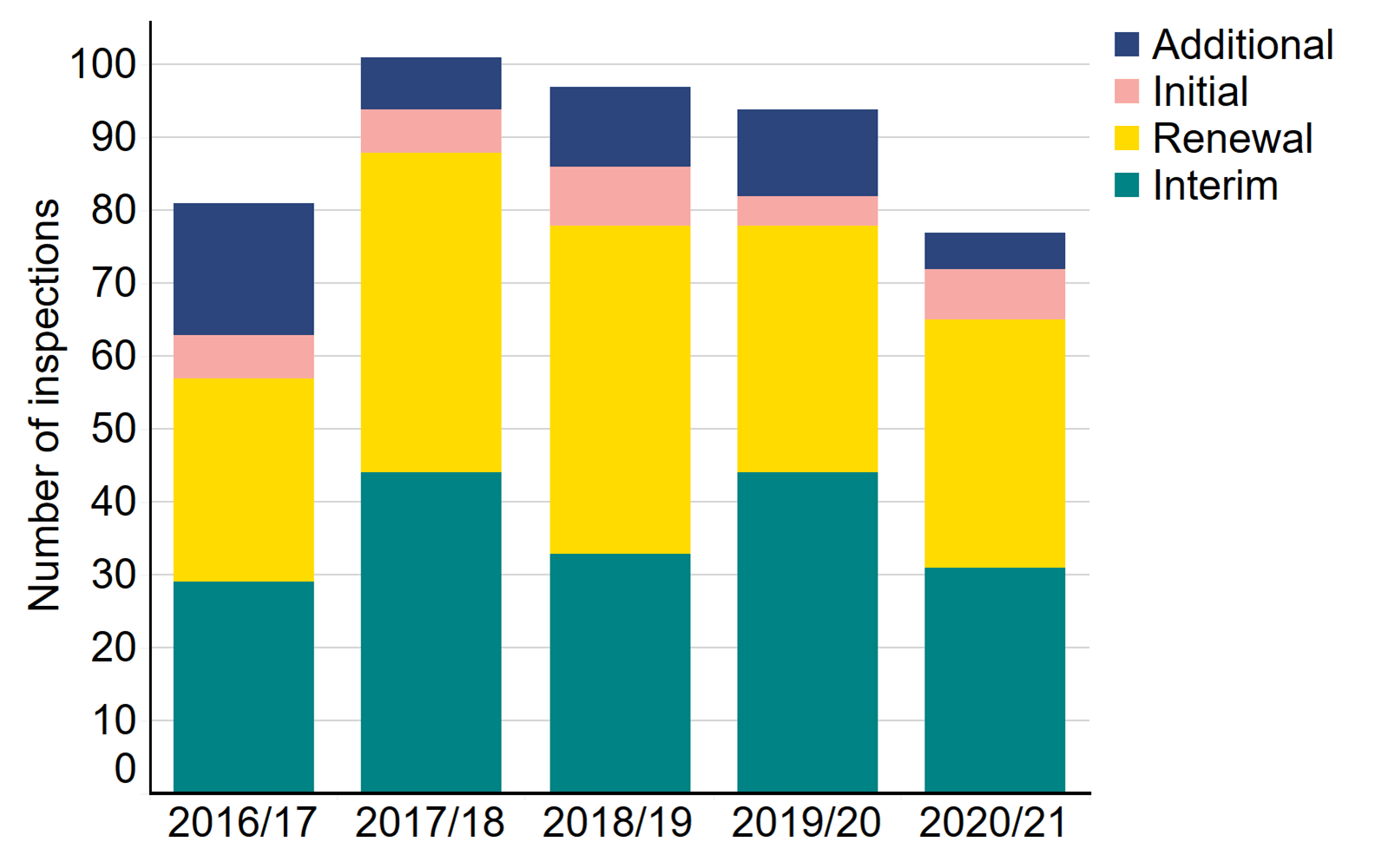
The table below shows the number of inspections conducted over the past five years. In 2020/21, 34 inspections (44%) were renewals, and 31 inspections (40%) were interim. Following each clinic inspection, a report identifying areas of good practice and those which are non-compliant and require improvement is published. In 2020/21, 63 non-compliances were identified during inspection. Due to the pandemic and the resulting modified approach to inspection, the numbers of onsite visits were reduced, and therefore the number of non-compliances is not comparable with previous years. The Clinical Governance Quarterly Updates provide clinics with detailed insight into non-compliances.
Number of inspections by type, 2016/17 - 2020/21
In 2020/21, 103 clinics were licensed by the HFEA to provide fertility treatment
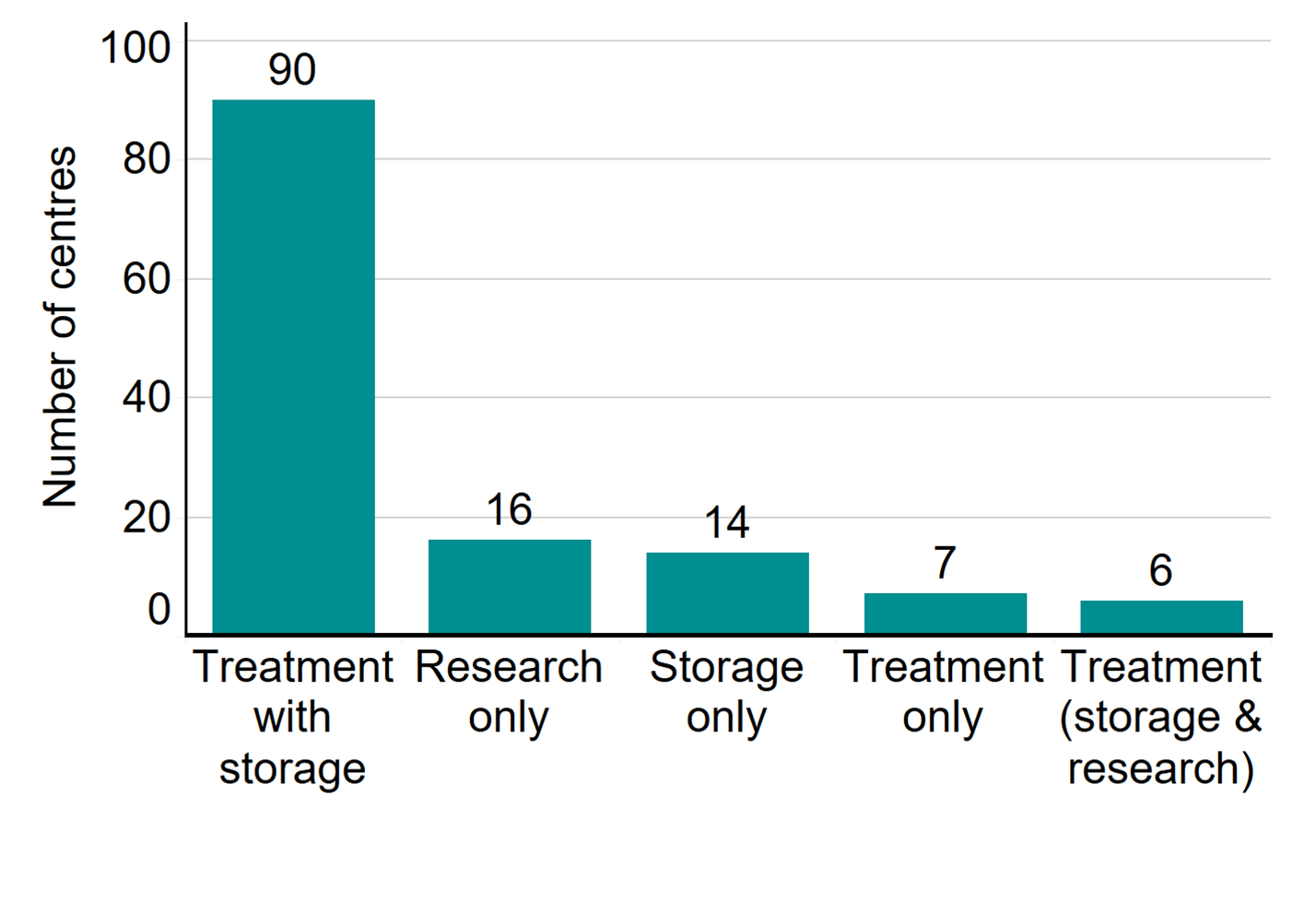
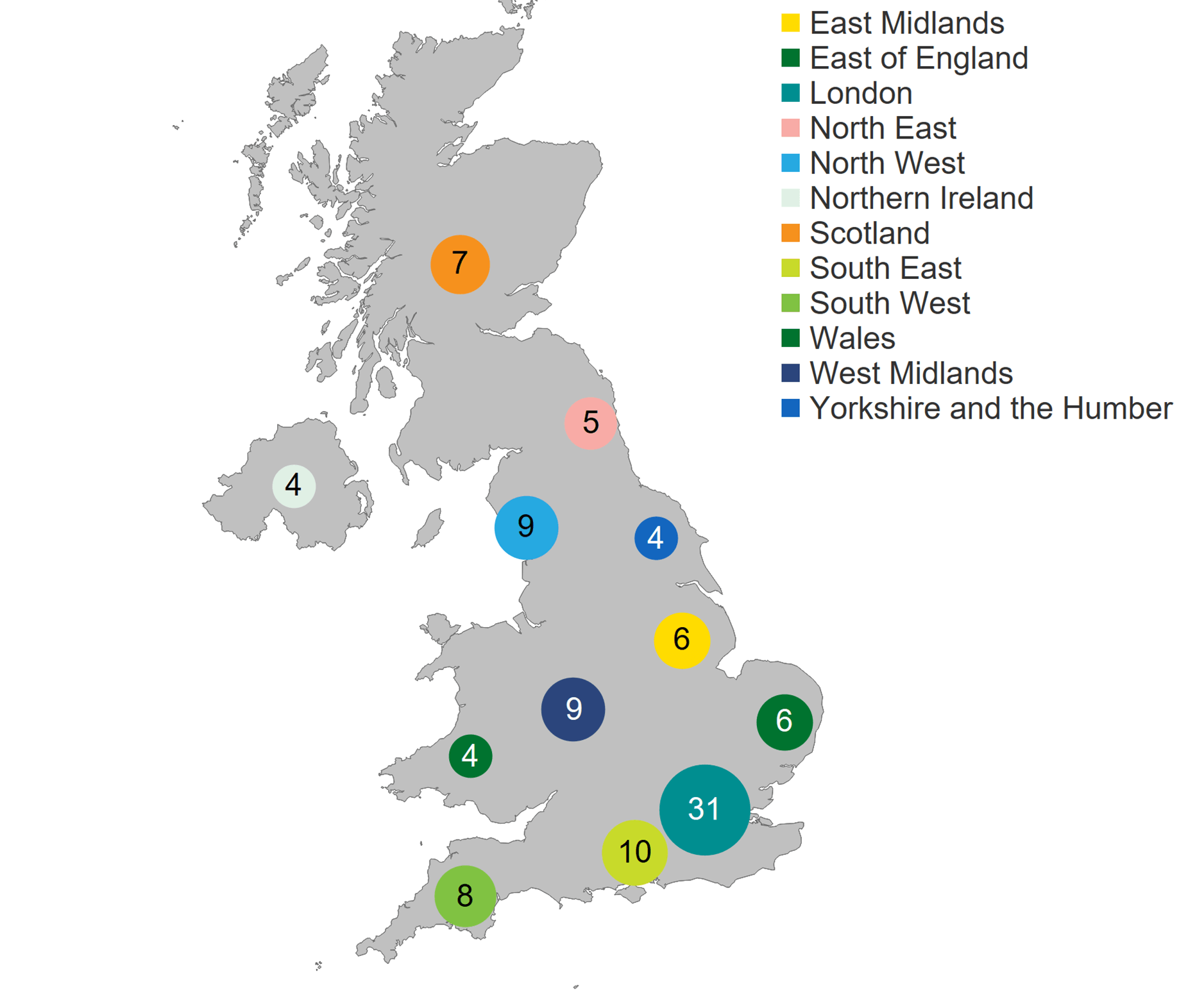
In 2020/21, there were 103 fertility clinics licensed by the HFEA in the UK to provide fertility treatment. In addition, 16 establishments were licensed to undertake research involving human embryos and 14 clinics were licensed to provide storage only. As in previous years, two-thirds of clinics were standalone, and the rest were owned by nine private clinic groups. Of the 103 licensed clinics that offered fertility treatment, 59 clinics (57%) are privately owned. The largest concentration of clinics offering fertility treatment were based in London (31 clinics), followed by the Southeast of England (10 clinics) and the West Midlands and the North West (9 clinics each).
Number of licensed fertility clinics by type, 2020/21
Number of clinics licensed to provide fertility treatment by geographical area, 2020/21 (excluding storage and research only)
The number of incidents in 2020/21 is broadly consistent with previous years
Each year, the vast majority of fertility cycles are carried out without any problems occurring. When incidents do occur in licensed fertility clinics, they must be reported to the HFEA in a timely manner. Proactive reporting allows clinics to learn from incidents and ensure changes can be made to prevent reoccurrence. We monitor incidents in clinics to make sure that everything is done to understand what went wrong and, crucially, to take steps to ensure it does not happen again. We also share learning and notify other clinics of potential issues to ensure all clinics learn from this. The Clinical Governance Quarterly Updates provide clinics with detailed insight into incidents.
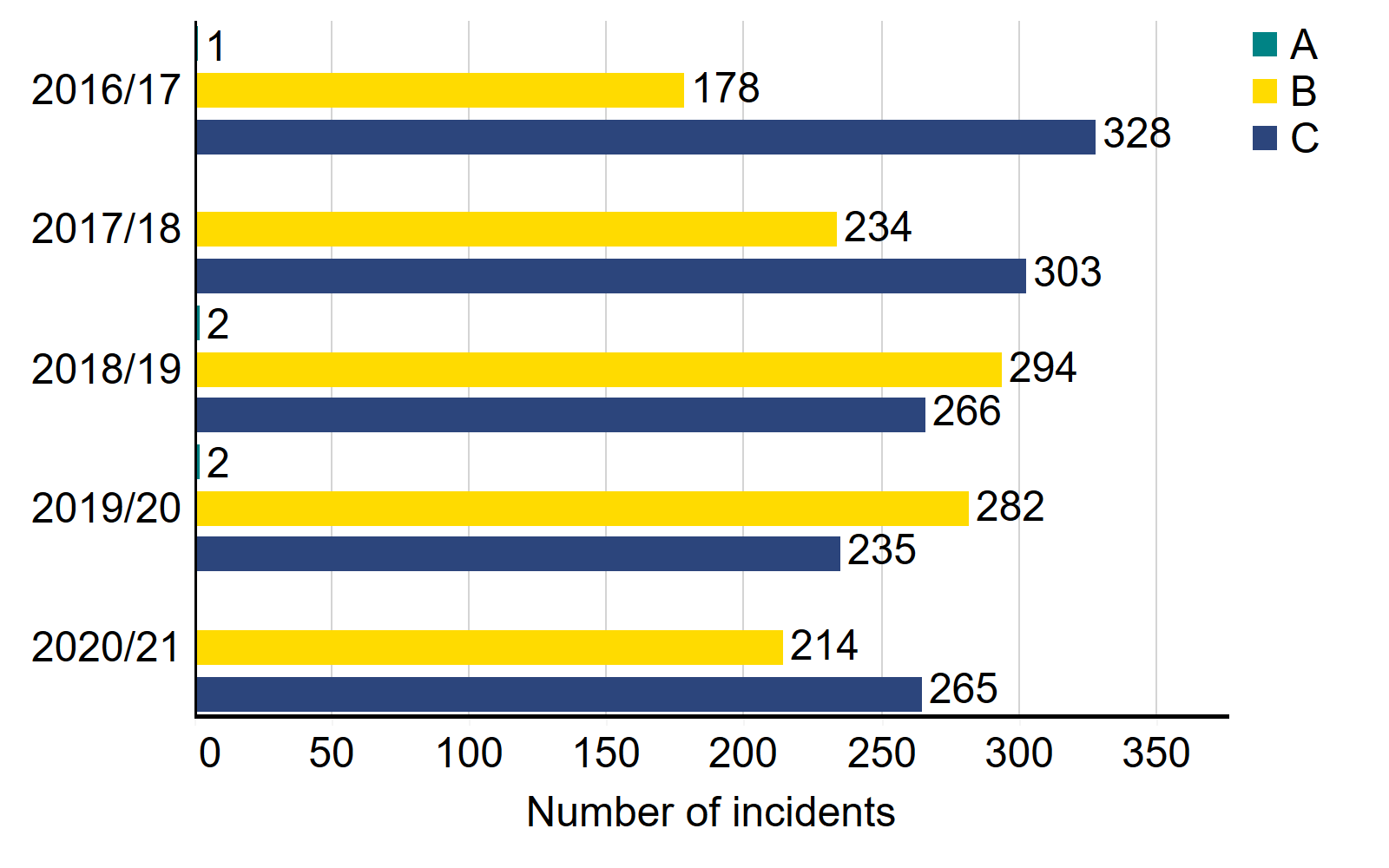
In 2020/21, 548 incidents (including near misses) were reported to the HFEA, compared to 569 in 2019/20. Although the overall number of incidents is broadly similar there were significant differences in the types of incidents reported to the HFEA: category C incidents increased by 13% in 2020/21 (265 incidents compared with 235 the previous year) and category B incidents continued to decline with 214 recorded in 2020/21, 24% fewer than in 2019/20.iii
Number of incidents by grade, 2016/17 – 2020/21
The number of severe incidents of OHSS reported declined in 2020/21
Ovarian Hyperstimulation Syndrome (OHSS) is a potentially serious side effect of the drug treatment necessary for fresh cycles of IVF, which unfortunately some patients develop. We have worked hard with clinics to ensure they do everything possible to prevent and manage OHSS. During the pandemic, clinics were encouraged to have an even more cautious approach to OHSS to reduce any additional burden on the NHS. This means that the number of severe cases of OHSS continues to decrease with 43 cases reported in 2020/21, a reduction of 35% on the previous year.
Number of severe OHSS incidents reported, 2016/17-2020/21
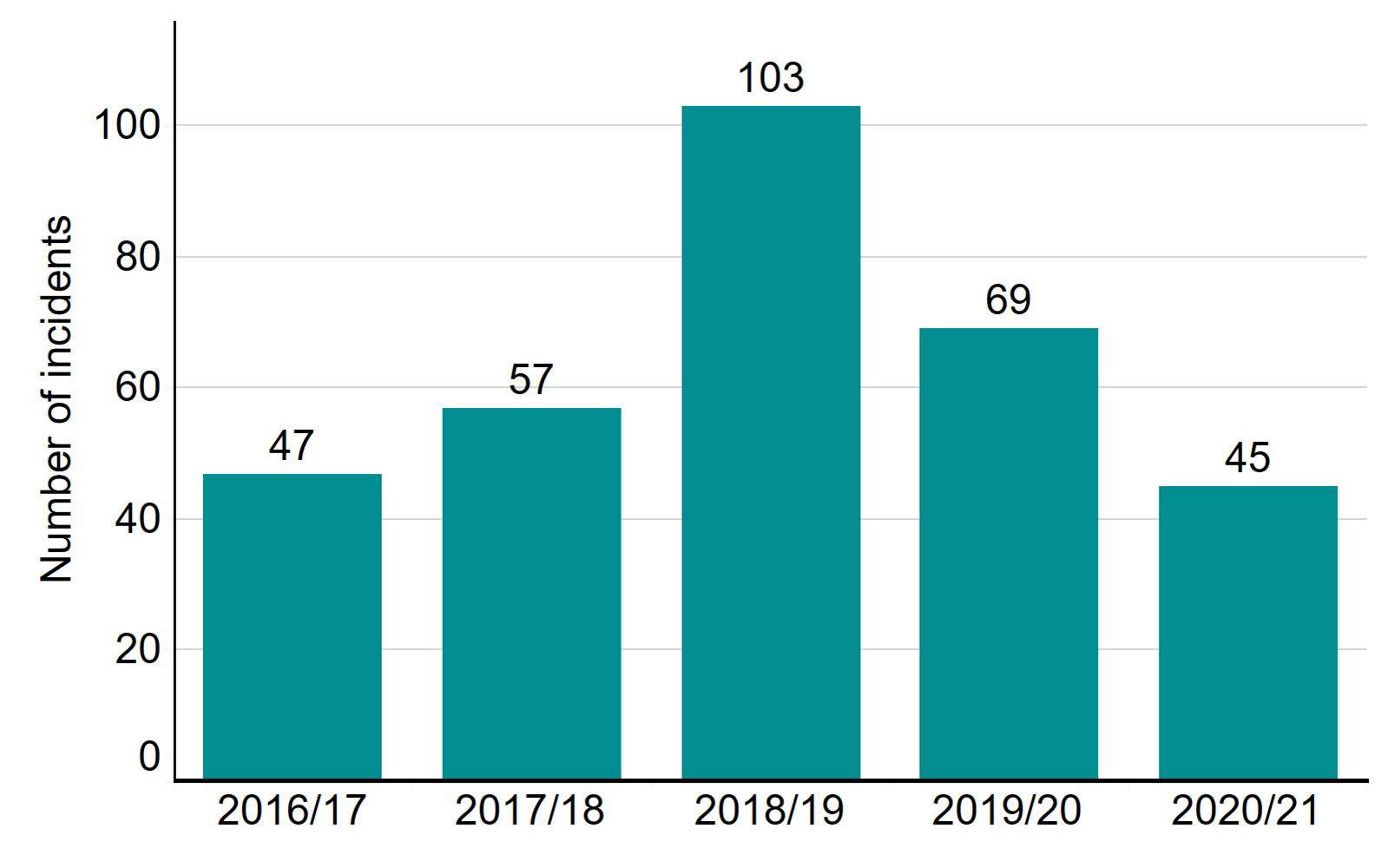
A total of 88 patient complaints were received about licensed clinics in 2020/21
Patients and donors can raise a complaint with us if they are unhappy with how a clinic has handled their original complaint. The number of patient complaints received in 2020/21 was 88, similar to the previous year, but the complexity of complaints has typically increased. Complaints received related to communication, lack of information and unclear or unexpected costs. We actively monitor and interrogate the information we receive regarding patient complaints and feed this into the inspection process. The Competition and Markets Authority has launched guidance on fertility treatment for clinics and patients.
Where a complaint is received, we discuss it with the clinic and encourage them to further engage with the complainant to try to locally resolve the issues raised. Complaints are discussed at regular clinical governance meetings to ensure clinics are actively engaging with patient complaints and providing thorough and well considered responses. We expect clinics to provide a sincere acknowledgement of the complainant’s experience, to explain what, if anything, went wrong, what measures the clinic has put in place to minimise the risk of this happening again, and what support the clinic can offer the complainant. The Clinical Governance Quarterly Updates provide clinics with detailed insight into complaints.
Further advice to clinics on thoroughly engaging and resolving patient complaints was included in a previous State of the Sector report and we would urge clinics to incorporate this into their complaint handling processes.
Number of complaints received, 2016/17-2020/21
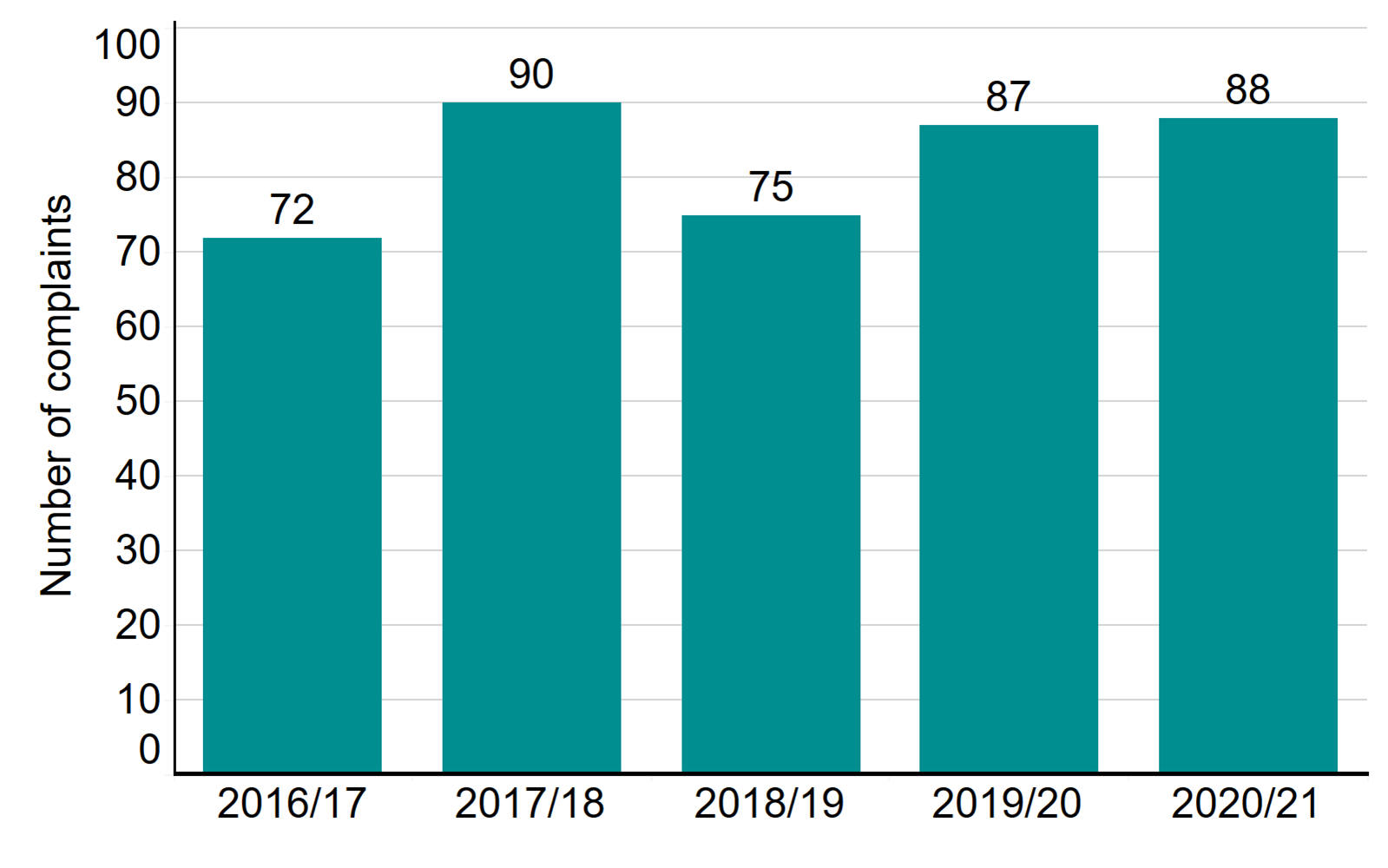
About our data
The information that we publish in this report is compiled from information gathered from our inspections throughout the year and other sources of information including our Register of fertility treatments, incident reports and patient complaints.
A full list of definitions of classification of incidents and complaints are available in the underlying dataset.
Treatment and patient numbers provided in Table 2 of the underlying dataset are published according to the year in which the cycle was started. An update to the underlying dataset was added in January 2024 to include details on the number of patients who underwent egg collection cycles, egg storage cycles and embryo storage cycles in 2019. Additionally, NHS and private IVF and DI cycles were updated and patient numbers for each added. All 2019 cycle and patient data provided in Table 2 of the underlying dataset are from our data warehouse containing Register data as of 10 January 2024.
Contact us regarding this publication
Media: press.office@hfea.gov.uk
Statistical: intelligenceteam@hfea.gov.uk
Notes on State of the Sector 2020/21
-
i General Direction 0014 Further background on this decision can also be found in the Authority papers from March – May 2021.
-
ii By issuing a revised General Direction 0014 (version 2)
-
iii Incidents are graded as:
- Grade A: involve severe harm to one person, or major harm to many
- Grade B: involves serious harm to one person, or moderate harm to many
- Grade C: involves minor harm
- Near miss: an event not causing harm, but has the potential to cause injury or ill health.
Review date: 26 February 2026

